Genomics, Justice, and Rights
I. The Belmont Report
II. Informed Consent
III. Gene Patenting
IV. Privacy of Biomaterials
The Belmont Report

A brief summary of the three main principles that are stated in The Belmont Report (“Belmont Report | HHS.gov,” n.d.) https://www.hhs.gov/ohrp/regulations-and-policy/belmont-report/
The Belmont Report was drafted by the National Commission in 1979. This report summarizes guidelines and ethical principles utilized in research. The three main principles that are provided by this report include Respect for persons, Beneficence, and Justice, as summarized in the infographic. Respect for persons promotes autonomy and that individuals with diminished autonomy should also be protected. Informed consent is an application of Respect for persons allowing individuals to know what will take place during the research. Beneficence is the principle that ensures that the benefits should be maximized and harm should be minimized when conducting research. Lastly, the Justice principle ensures that the distribution of burdens and benefits are fairly shared (“Belmont Report | HHS.gov,” n.d.). Overall the Belmont report goes hand in hand with our perspective and the ethical issues we will be discussing. To see the full Belmont Report, click here.
Informed Consent
Informed Consent is the process of acquiring permission from subjects who are potentially participating in research. Before a participant enters an experiment, there are a series of formalities that must occur. This enables patients to be fully aware of the parameters of the study before they agree to enter into it. The Institutional Review Board reviews each study. A participant has the right to call them at any time to express concerns they may have about the study. The experimenters must disclose all the rights the participant has, a few of which include that the participant can remove themselves at anytime, they have a right to know the outcome and any new information gained during the study, and the potential risks of participating. For more information click here, or watch this short video.
The dilemma of informed consent resides around the first ethical principle outlined in the Belmont report: respect for persons. Giving research subjects informed consent is an application of that principle. Tension lies between the utilitarian outcome of research doing the greatest good if informed consent wasn’t applied, and the full privacy of research subjects. It is difficult to accomplish both. Despite the moral necessity that may come with consent, if complete consent was granted every time a specimen was removed and/ or used for something that was not previously mentioned to said subject, scientific growth and development could be inhibited as a result. Conversely, the rapid progression of science technology is why there is a call for informed consent and non-physical consequences and secondary tissue usage should be outlined in it; there is no true way to remain anonymous anymore which poses the threat of infringement on privacy and thus respect of persons. In the chart below, the harms and benefits of the use (or potential use) of informed consent are outlined for prominent examples of controversial research.
Gene Patenting
A Brief History of Gene Patenting in the U.S
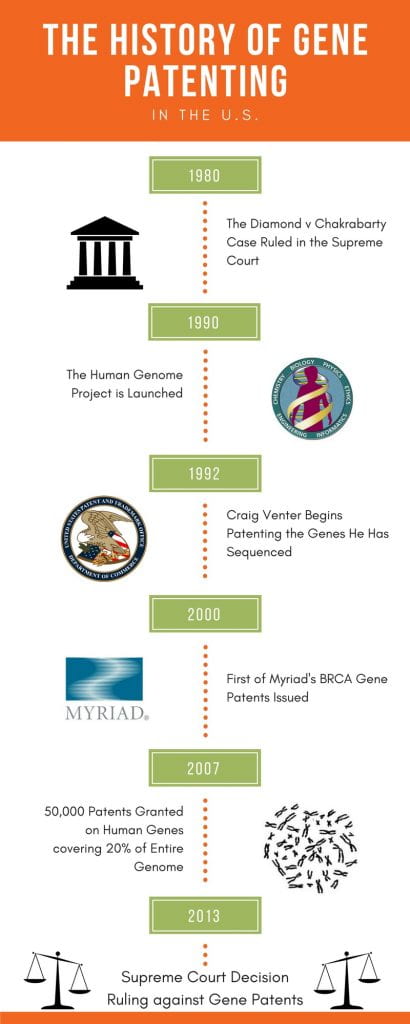
A brief timeline of Gene Patenting in the U.S. (Council for Responsible Genetics, 2009; Diamond v. Chakrabarty, 1980; Yang, 2009)
The story of how gene patenting began in the U.S. gives an insight into the complexity of this issue and why it was first considered acceptable. In 1980, the U.S. Supreme Court upheld the case of Diamond vs. Chakrabarty, in which it was decided that “a live, human made micro-organism is patentable subject matter” (Diamond v. Chakrabarty, 1980). This was the first major precedent set to allow the patenting of biotechnology discoveries and it opened the door for the earliest genetic patents, issued in 1982 (“Intellectual Property and Genomics,” 2014). Not long after this, Craig Venter, one of the head researchers and founders of the HGP, developed a new method for sequencing genomes while working at the NIH. A lawyer quickly alerted Venter that, due to the Bayh-Dole Act, he would need to seek a patent on the genes he sequenced before publication because they could be commercially valuable (Yang, 2009). The practice of patenting genes continued from then on, including several controversial patents, such as those filed by Myriad Genetics for the BRCA genes, beginning in 2002. By 2007 more than 50,000 patents had been filed, covering over 20% of the entire human genome (Council for Responsible Genetics, 2009). Finally, in 2013 the U.S. Supreme Court ruled that DNA in its natural form cannot be patented because nothing new was created, and therefore no intellectual property existed (“Intellectual Property and Genomics,” 2014).
Ethical Issues On The Local Scale

Plowing a field could be used as grounds for legal ownership of land, as work has been invested in the land. Permission for use of image granted by Roletschek (Quigley, 2012; Roletschek, 2004).
One of the first ethical concerns is whether part of the human body even can be deemed patentable (Skloot, 2011). In the case of human genes, lots of work went into the isolation of these genes, especially as the technology was first developing, and work has been used as grounds for patents in the past (Quigley, 2012). However, the first person to uproot a tree was not allowed to claim that they then owned every single tree of that species across the country, and attempt to spread that ownership across the globe. It is a violation of our natural rights as humans to give one person a piece of every human as property, regardless of work that has gone into isolating it.
Another problem is the incentives that naturally come along with patents. Specifically, the encouragement of innovation in owning an initial property and the requirement that that information is made public for others to use as they could. In the case of patenting human genes, the exact opposite happens—these patents make it impossible for anyone other than the patent holder to conduct research on that gene, allowing for no potential gain in public innovation on the patented gene (Jamison, 2015). In this case, patents are seen disrupting scientific progress. In terms of utilitarian ethics, for the greatest good of the people, science should not be disrupted.
A third issue comes with the patenting of specific gene mutations that can confer an inherited risk to certain forms of cancer or other diseases. This is especially highlighted in the Case of Myriad Genetics.
The Case of Myriad Genetics
The story of Myriad Genetics and the patenting of the BRCA1 and BRCA2 genes goes back to the early 1990s where a fierce competition to discover genetic factors leading to breast cancer was taking place. Myriad Genetics was able to first sequence and patent the BRCA1 gene in 1994 (Gold & Carbone, 2010). Myriad Genetics was then able to beat a team of researchers from the U.K. in sequencing and patenting the BRCA2 gene in 1995 (Gold & Carbone, 2010). The company has been challenged multiple times as to whether they actually earned the patents and if their gene patents should be allowed. The American Civil Liberties Union along with researchers and breast cancer survivors sued Myriad Genetics in 2009 as they believed the gene patents were inhibiting research and scientific progress (Skloot, 2017). Myriad Genetics was causing such a stir that the supreme court heard the case of the Association for Molecular Pathology v Myriad Genetics and on June 13, 2013 the court ruled, “(1) that isolated genomic DNA (gDNA) is not patent-eligible under section 101 of the Patent Act, but (2) cDNA is,” bringing the issue to a close (Conley, 2013).
The issues surrounding the patenting of these genes and resulting diagnostics bring about many ethical considerations. A popular argument in the case of gene patenting is that gene patenting spurs innovation, as seen in the competition to sequence and patent the BRCA1 and BRCA2 genes. However, while this holds some truth, the fact that patents prevent further, possible life saving, research more than likely outweighs the benefits of competition for such patents. Another ethical issue that results from gene patenting is the complete control of diagnostics for the patented genes, as seen in the Myriad Genetics case. Since only Myriad Genetics can sell the diagnostic kits for breast and ovarian cancer, they are able to sell the test at a higher cost than if multiple companies were producing the test. The result of this is an inability to pay for the test by those lacking adequate funds. This is unjust as it prevents equal health coverage for all people, allowing people with more money access to better diagnostics which could be life saving.
Ethical Issues On The Global Scale
These issues occur everywhere that human genes are patentable. Some nations have made progress and made patenting human genes illegal, however there are still many places in the world that it is still legal. Across the world, it is illegal in U.S. as of 2013 (“Intellectual Property and Genomics,” 2014) and Australia as of 2015 (Slezak, 2015)—though it was initially upheld in 2013 (Corderoy, 2013), legal in Canada (Ubelacker, 2016) and the 41 states under the EPO (Sharples, 2011), and it can be legal but the language of the law makes it more difficult in Japan (Chen & Sugimura, 2013).
This international disagreement on whether human genes can be patented brings about new ethical concerns. Patents are supposed to give the patent holder the potential for growth with capital gained from their property, but with international disagreement, patent holders may be unsure of the extent of their patent protection in different jurisdictions and could potentially avoid those jurisdictions out of caution. This is especially harmful to smaller businesses, where their patents are essential for growth. Unclear patent protection in given jurisdictions can also harm competitors who may invest money in research on the same subject matter hoping that the patent will not extend to their jurisdiction, but then it does and they have wasted their money (Jamison, 2015). This again shows how human gene patenting has harmed people and hindered scientific progress.

The legal status of patenting of human genes in countries of interest. Information was found on the U.S., Canada, 41 countries that fall under the European Patent Office (EPO), Australia, and Japan. Countries highlighted red indicate that patenting is illegal, green indicates patenting is legal, and blue indicates patenting is legal but more difficult (Camphire, 2017).
Privacy of Biomaterials
A huge debate related to tissue privacy across America is newborn blood screening. Hours after a baby is born, a small blood sample is drawn and sent off to a lab to be tested. The benefit of doing this is to identify life-threatening diseases before symptoms begin to show, that way doctors can begin treatment
A newborns blood is getting screened for rare diseases. “Privacy in Genomics.” National Human Genome Research Institute (NHGRI). NIH, 21 Apr. 2015. Web. 18 Feb. 2017. <https://www.genome.gov/27561246/privacy-in-genomics/>.
immediately. (Privacy in Genomics) Furthermore, the samples can be used to examine disease rates among different regions, races, and ethnicity. This gains valuable data about disease prevalence in different populations, but that’s not always fully disclosed. (Tarini 2001) Is it fair to parents that procedures other than the blood screen, such as selling tissues to biotech companies, using them for research, or storing them in a bio-bank for as many as 23 years in some states could be going on using their child’s blood, and they have no idea it is happening? (Brase 2017) Numerous studies show not many parents even remember the screen taking place, and even fewer can recall the reason for the screening, highlighting the lack of awareness regarding the program. The program is on the verge of become a “victim of its own success” due to the rise in concern of privacy and disclosure problems (Tarini 2001). Common Rule, plays a big part in protecting human subjects who participate in research. It outlines a basic set of rights that need to be respected during research, and requires informed consent. (Privacy and Confidentiality) The Newborn Screening Saves Lives Act Re-authorization act allows screening for life threatening disease, while requiring consent if the blood spots collected will be used in research (Privacy in Genomics). While these policies help protect patients who may otherwise be taken advantage of, it is still our responsibility as citizens to question if it is ethically correct to carry out a research task, especially when there are few laws governing the practice. This practice violates informed consent, when babies blood is being tested for things other than parents were informed of. This is a issue of rights, and consent, especially when the initial test is taken without the parents knowledge.
About the Authors
Brooke, Colin, Rachel, Sabrina, Shaw, and Tryphena are studying the ethical and social issues within tissue ownership. Together they make up the Bioethics Group in their ISAT 456 course, Ethical, Legal, and Social Implications of Biotechnology, in one of the largest schools in Virginia, James Madison University.
Disclaimer: This was a collaborative class project that represents multiple viewpoints. Some views may not be shared by all contributors to this page
Works Cited
[AbsoluteResearch]. (2014, March 9). What is Conformed Consent. Adult. [Viedo File]. Retreived from https://www.youtube.com/watch?v=OrAA9Lq9xKU.
Brase, Twila. “Parents and Children Deserve Genetic Privacy.” U.S. News and World Report, 28 Apr. 2014. Web. 17 Feb. 2017. <http://www.usnews.com/opinion/articles/2014/04/28/newborn-screenings-violate-dna-privacy-rights>.
Camphire, S. G. (2017, February 16). Legal Status of Gene Patenting in Countries of Interest [Digital image].
Chen, R., & Sugimura, K. (2013). IPS Cell Technology Spurs Biological Patenting in Japan. London, England.
Commins, J., Toft, C., & Fares, M. A. (2009, March 11). Whole genome shotgun sequencing versus Hierarchical shotgun sequencing [Digital image].
Cooke-Deegan, R. (2008). Gene Patents. In From Birth to Death and Bench to Clinic: The Hastings Center Bioethics Briefing Book for Journalists, Policymakers, and Campaigns (pp. 69–72). Garrison, New York: The Hastings Center.
Corderoy, A. (2013, February 15). Landmark Patent Ruling Over Breast Cancer Gene BRCA1. The Sydney Morning Herald. Sydney, Australia.
Council for Responsible Genetics. (2009). Scope and Cost of Gene Patenting in the United States. Cambridge, MA.
[Empowered Doctor]. (2016, Feb 23). The Ethical Implications of Gene Patenting. [Video File]. Retrieved from https://www.youtube.com/watch?v=9QVv0ugtJjw.
Gold, E. R., & Carbone, J. (2010). Myriad Genetics: In the eye of the policy storm. Genetics in Medicine,12(4). doi:10.1097/gim.0b013e3181d72661
Intellectual Property and Genomics. (2014).
Jamison, M. (2015). Patent Harmonization in Biotechnology: Towards International Reconciliation of the Gene Patent Debate. Chicago Journal of International Law, 15(2), 688–720. Retrieved from http://chicagounbound.uchicago.edu/cjil/vol15/iss2/9
“Privacy and Confidentiality.” Current Issues in Research Ethics : Privacy and Confidentiality. N.p., n.d. Web. 20 Feb. 2017. <http://ccnmtl.columbia.edu/projects/cire/pac/foundation/#1>.
“Privacy in Genomics.” National Human Genome Research Institute (NHGRI). NIH, 21 Apr. 2015. Web. 18 Feb. 2017. <https://www.genome.gov/27561246/privacy-in-genomics/>.
Roletschek, Ralf. (2004, September 12). Farmer plowing field in Germany [Digital Image].
Sharples, A. (2011, March). Gene Patents in Europe Relatively Stable Despite Uncertainty in the U.S. GEN, 23. Retrieved from http://www.genengnews.com/gen-exclusives/gene-patents-in-europe-relatively-stable-despite-uncertainty-in-the-us/77899385
Skloot, R. (2017). The Immortal Life of Henrietta Lacks. S.l.: Broadway Books.
Slezak, M. (2015, October 7). Genes Can’t Be Patented, Rules Australia’s High Court. New Scientist. Retrieved from https://www.newscientist.com/article/gene-patents-struck-down-by-australias-high-court/
Tarini, Beth A., and Aaron J. Goldenberg. “Ethical Issues with Newborn Screening in the Genomics Era.” Annual Review of Genomics and Human Genetics. U.S. National Library of Medicine, 21 May 2001. Web. 22 Feb. 2017. <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3625041/>.
“The Belmont Report.” HHS.gov. U.S. Department of Health and Human Services, 15 Mar. 2016. Web. 01 Mar. 2017.
Quigley, M. (2012). Property in Human Biomaterials— Separating Persons and Things? Oxford Journal of Legal Studies, 32(4), 659–683.
Ubelacker, S. (2016, March 20). Status of Gene Patents in Canada Unresolved, Despite Successful Challenge. CTV News. Toronto. Retrieved from http://www.ctvnews.ca/health/status-of-gene-patents-in-canada-unresolved-despite-successful-challenge-1.2824957
Yang, B. (2009). Executive Summary of a Book — The Genome War: How Craig Venter Tried to Capture the Code of Life and Save the World. Discovery Medicine.

