DESCRIPTION:
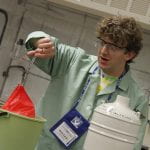
When a balloon is placed in liquid nitrogen the air inside it is condensed from the cold (-196°C), causing the balloon to shrink. Once the balloon is removed it will regain its size as the air heats up. Liquid nitrogen boils at room temperature. The “fog” that we see is condensed water vapor though, not nitrogen gas.
TOPICS COVERED:
– cryogenics
– gas laws
– physical change
– vaporization
– condensation
MATERIALS NEEDED:
– liquid nitrogen
– blown up balloon
– tongs
PROCEDURE:
1. Place the balloon in the liquid nitrogen
2. Once it is shrunk, use the tongs to place it on the bench top
ADDITIONAL COMMENTS:
This is a great one to do in front of an audience or as a hands on activity.
SAFETY:
Liquid nitrogen is a cryogenic hazard and cause severe skin burns. Safety goggles should be worn at all times.
STORY:
Bet the students you can shrink a balloon without touching it or popping it.
This problem set practices unit conversions, calculating volume and density, and using the Ideal Gas Law.
This lesson plan‘s topic is the three gas laws: Charles’, Boyle’s & Avogadro’s. Each of these laws is examined in the context of balloons. Shrink a Balloon is the hook in this lesson.