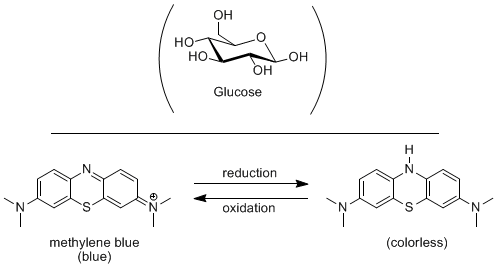
DESCRIPTION:
Under basic conditions glucose is oxidized while methylene blue is reduced, changing from blue to colorless. The reduction reaction can be reversed by shaking the flask which forces oxygen into solution, which oxidizes the methylene blue, thus turning it back to blue. If the flask is let sit, the oxygen will come out of solution making the solution colorless and ready to shake again.
TOPICS COVERED:
– redox
– indicators
– organic reactions
– color change
– chemical change
– equilibrium
MATERIALS NEEDED:
– KOH
– glucose
– 1% methylene blue solution
– water
– 125mL Erlenmeyer flask
– stopper
– parafilm
– beaker
– graduated cylinder
PROCEDURE:
1. Dissolve 4g of KOH into 40mL of water in the beaker
2. Dissolve 4g of glucose into 40mL of water in the flask
3. Add 2-4 drops of methylene blue to the flask
4. Pour the KOH solution into the flask
5. Stopper and wrap parafilm around the stopper
6. Wait for the solution to go clear, then shake
ADDITIONAL COMMENTS:
This is a great one to prep ahead of time, then take to lecture. The flask should last for a few shakes.
SAFETY:
Safety goggles should be worn at all times. KOH can cause burns if handled.
REFERENCES:
Shakhashiri, B.Z. Chemical Demonstrations; University of Wisconsin Press: Madison, 1985; Vol. 2, pp 142.
“Blue Bottle Experiment.” Union College Chemistry. Accessed 4 July 2010.
http://www.union.edu/academic_depts/chemistry/faculty/fox/Chemical%20Demonstrations/docs/10%20Blue%20Bottle.pdf
This lesson plan‘s topic is the Introduction of Chemical Reactions. This lesson introduces students to the symbols associated with reactions, using this PowerPoint, and has a short inquiry style lab shows students the Law of Conservation of Mass. Blue Bottle is an example of a reversible reaction in this lesson.