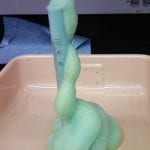
DESCRIPTION:
Hydrogen peroxide is decomposed quickly in liquid soap with the help of a catalyst to create a large volume of foam that grows out of a graduated cylinder. The soap bubbles contain oxygen.
2H2O2 → 2H2O + O2, KI is a catalyst
TOPICS COVERED:
– decomposition reactions
– catalysis
– exothermic reactions
– evolution of a gas
– color change
– chemical change
MATERIALS NEEDED:
– 100-mL graduated cylinder
– 10-mL graduated cylinder
– 30% H2O2 solution
– 2 M KI
– dish soap
– food coloring (optional)
– tray to contain the foam in
PROCEDURE:
1. Place the 100-mL graduated cylinder in the tray
2. Add ~ 3 mL of dish soap to the graduated cylinder
3. Add 10 mL of H2O2
4. Add food coloring, and swirl to stir
5. Measure out 5 mL of KI in the 10-mL graduated cylinder
6. Add the KI to the large cylinder
ADDITIONAL COMMENTS:
Without food coloring the foam takes on a yellow brown color. Adding more peroxide will increase the volume of foam (increase the amount of soap too.) Adding more/less KI will increase/decrease the reaction rate. This reaction can also be done in a 100-mL volumetric flask, but the flask is more difficult to rinse completely than the graduated cylinder. Baquacil (a pool oxidizer) can be used instead of 30% H2O2.
SAFETY:
The 30% H2O2 used in this demonstration is a strong oxidizing agent which can damage the eyes, skin and respiratory tract, so wear gloves and always wear safety goggles.
REFERENCES:
“Elephant’s Toothpaste.” Steve Spangler Science. Accessed 25 Jan 2010.
http://www.stevespanglerscience.com/experiment/hydrogen-peroxide-eruption
STORY:
– For elementary students we typically begin by asking students what kind of pets they have, then say that we have a pet elephant. Our pet elephant is so big that he needs a lot of toothpaste which gets expensive, so we make our own.
– For older students we typically explain that when you are in college you are always broke, so you don’t want to have to spend money on basic things like toothpaste. Instead we make toothpaste for ourselves and in this case, it’s enough for the entire hall.
This lesson plan‘s topic is the Evidences of Chemical Reactions. Students learn the various evidences, then they get a change to practice identifying them with a hands on activity. Elephant’s Toothpaste is used in the notes portion of this lesson.