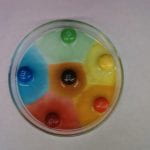
DESCRIPTION:
When M&M’s are placed in water, the outer shell, which is made of sugar, dissolves. The sugar moves from a place of high concentration (the M&M) to a place of low concentration (the water away from the M&M). When the sugar shell dissolves and moves outward, it takes the layer of food dye with it. When more than one M&M is placed into a petri dish the colors do not mix because the concentration of sugar at the interface is approximately the same. Also, around the bottom of the M&M water appears cloudy because the partially dissolved sugar is more dense than the water, so it sinks.
TOPICS COVERED:
– density
– dissolution
– diffusion
– physical change
– solubility
MATERIALS NEEDED:
– petri dish
– white paper
– water
– M&Ms
PROCEDURE:
1. Place the petri dish on white paper
2. Add a small amount of water to the petri dish
3. Place M&Ms at the edges of the petri dish, equally spaced around the dish
ADDITIONAL COMMENTS:
This demo would work great on an overhead. Different patterns can be made by placing the M&Ms in different places around the dish and by using different colors.
SAFETY:
Safety goggles should be worn at all times.
REFERENCES:
Meerman, Ruben. “Cool Colour Experiment.” Posted 20 June 2007. Accessed 17 June 2010. http://www.abc.net.au/science/surfingscientist/pdf/lesson_plan15.pdf